Background: The combination of a hypomethylating agent (HMA) plus venetoclax is the standard of care for patients (pts) with acute myeloid leukemia (AML) who are older (age ≥ 75 yrs) and/or unfit for intensive chemotherapy. ASTX727 (oral decitabine/cedazuridine) is an oral formulation of decitabine with pharmacokinetic AUC equivalent exposures to IV decitabine. We performed a phase 2 study to evaluate the efficacy and safety of ASTX727 plus venetoclax in the frontline (FL) and relapsed-refractory (R/R) settings.
Methods: Pts ≥ 18 yrs with WHO 2016-defined AML were eligible. The FL cohort enrolled newly diagnosed pts with ECOG ≤ 3 who were either ≥ 75 yrs or ineligible for intensive chemotherapy due to comorbidities. Pts ≥ 18 yrs and ECOG ≤ 2 were eligible for the R/R cohort. Pts with a history of myelodysplastic syndrome (MDS), including previously treated MDS, were eligible. Treatment consisted of ASTX727 (decitabine/cedazuridine) 35/100 mg PO daily x 5 days and venetoclax 400 mg PO daily (adjusted for azoles) for 28 days during induction (held if blast clearance on day 21 bone marrow examination) and 21 days in consolidation. Dose reductions were permitted for toxicity/tolerability. Therapy was administered in 28-day cycles for up to 24 cycles. Risk stratification and responses were determined per ELN 2022. This study was registered on ClinicalTrials.gov (NCT04746235).
Results: 52 pts have been enrolled (42 in the FL and 10 in the R/R cohorts). In the FL cohort, the median age was 79 yrs (range 50-92). 17/42 (40%) pts had antecedent MDS (including 8 [19%] with treated secondary AML) and 6/42 (14%) had therapy-related AML (t-AML). By ELN 2022, 6 (14%) were favorable, 3 (7%) were intermediate, and 33 (79%) were adverse. 9/42 (21%) had complex cytogenetics and 6 (14%) had TP53 mutations. In the R/R cohort (n=10), the median age was 71 yrs (46-75). 2 (20%) pts had antecedent MDS and 2 (20%) had t-AML. By ELN 2022, 3 (30%) were intermediate and 7 (70%) were adverse. 5/10 (50%) had complex cytogenetics and 2/10 (20%) had TP53 mutations. The median prior lines of therapy in the R/R cohort was 2 (1-4), with 5 (50%) pts with 2 or more prior lines of therapy.
The median number of cycles given was 3 (1-12). In the FL cohort, the ORR was 67% (28/42 pts: 15 [36%] CR, 11 [26%] CRi, 2 [5%] MLFS). In responding FL pts, measurable residual disease (MRD) by flow cytometry became undetectable in 7/22 (32%) pts with adequate samples. In the R/R cohort, the ORR was 50% (5/10 pts: 3 [30%] CR, 2 [20%] CRi). The median number of cycles to first and best response were 1 (1-4) and 1 (1-7), respectively.
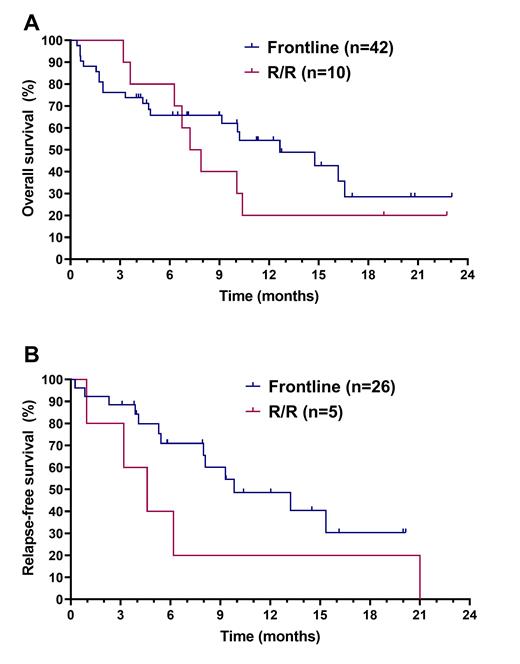
At a median follow-up of 12.8 months, the median OS was 12.7 months in the FL cohort and 7.6 months in the R/R cohort (figure A). In pts achieving CR/CRi (n=26 FL and 5 RR), the median RFS was 9.8 months in the FL and 4.6 months in the R/R cohort (figure B). The median DOR was 13.2 and 4.6 months the FL and R/R settings, respectively. 2 (4%) pts (1 FL and 1 R/R) went off protocol to undergo stem cell transplantation.
We then stratified the FL pts into 3 genetic groups (group 1: TP53 wt, K/NRAS wt, no FLT3-ITD; group 2: TP53 wt, K/NRAS mut or FLT3-ITD positive; group 3: TP53 mut) as previously described (Döhner, ASH 2022). The median OS in group 1 (n=24), group 2 (n=12), and group 3 (n=6) was 16.2, 9.1, and 1.4 months, respectively. In FL pts achieving CR/CRi, the median RFS in group 1 (n=17), group 2 (n=7), and group 3 (n=2) was 13.2, 8.1, and 9.8 months, respectively. OS in the FL cohort was similar between the pts under 80 yrs (n=21, median 12.7 months) vs those 80+ yrs (n=21, median 14.8 months), p=0.98.
The most common grade 3/4 adverse events were neutropenic fever (23%), pneumonia (13%), bacteremia (8%), cellulitis (6%), sepsis (6%), and respiratory failure (6%). 4-wk and 8-wk mortality were 10% and 15%, respectively. The median time to cycle 2 was 38 days (26-70). In pts who experienced count recovery during cycle 1, the median time to platelets > 50 x 10 9/L and absolute neutrophil count > 1.0 x 10 9/L was 23 (18-55) and 42 (28-69) days, respectively. Cycle 2 dose reductions occurred in 27/42 (64%) pts (21 venetoclax dose reductions, 6 both drugs reduced). Of the 29 observed deaths, 15 (52%) occurred in non-responders, 11 (38%) following AML relapse, and only 3 (10%) in pts with ongoing response (1 sepsis, 1 GI hemorrhage, and 1 electing to pursue hospice).
Conclusions: In this older and very high-risk population of pts with AML, an entirely oral regimen consisting of ASTX727 and venetoclax was effective and tolerable.
OffLabel Disclosure:
Garcia-Manero:Genentech: Research Funding; Bristol Myers Squibb: Other: Medical writing support, Research Funding; AbbVie: Research Funding. Short:Stemline therapeutics: Research Funding; Amgen: Honoraria; Astellas: Research Funding; Takeda: Consultancy, Research Funding; AstraZeneca: Consultancy; Novartis: Consultancy; Pfizer: Consultancy. Alvarado Valero:Astex: Research Funding; CytomX Therapeutics: Consultancy; Sun Pharma: Consultancy, Research Funding; BerGenBio: Research Funding; Jazz: Research Funding; MEI Pharma: Research Funding; Daiichi-Sankyo: Research Funding; FibroGen: Research Funding. Issa:Celgene: Research Funding; Kura Oncology: Consultancy, Research Funding; NuProbe: Consultancy; Syndax: Research Funding; Novartis: Consultancy, Research Funding; Merck: Research Funding. Maiti:Celgene: Research Funding; Lin BioScience: Research Funding. Yilmaz:Pfizer: Research Funding; Daiichi-Sankyo: Research Funding. Jain:MEI Pharma: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Aprea Therapeutics: Research Funding; Fate Therapeutics: Research Funding; Beigene: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; TG Therapeutics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; ADC Therapeutics: Research Funding; AstraZeneca: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; TransThera Sciences: Research Funding; Takeda: Research Funding; Mingsight: Research Funding; Novalgen: Research Funding; Loxo Oncology: Research Funding; Medisix: Research Funding; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; AbbVie: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Servier: Research Funding; Pharmacyclics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Kite/Gilead: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; BMS: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Precision Biosciences: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Ipsen: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; CareDX: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Pfizer: Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Incyte: Research Funding; Cellectis: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Genentech: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Newave: Research Funding; Dialectic Therapeutics: Research Funding. Masarova:MorphoSys US: Membership on an entity's Board of Directors or advisory committees. Jabbour:Pfizer: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Hikma Pharmaceuticals: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Adaptive Biotech: Consultancy, Honoraria, Research Funding; Ascentage Pharma Group: Consultancy, Honoraria, Research Funding. Montalban-Bravo:Takeda: Research Funding; Rigel: Research Funding. DiNardo:Notable Labs: Honoraria; Servier: Honoraria; AbbVie/Genentech: Honoraria; Astellas: Honoraria; Fogham: Honoraria; ImmuniOnc: Honoraria; Novartis: Honoraria; Takeda: Honoraria; Schrödinger: Consultancy; BMS: Honoraria. Kadia:GenFleet Therapeutics: Research Funding; Cure: Speakers Bureau; Genzyme: Honoraria; Agios: Consultancy; Liberum: Consultancy; Cyclacel: Research Funding; Cellenkos Inc.: Research Funding; Ascentage Pharma Group: Research Funding; Jazz Pharmaceuticals, Pfizer, Pulmotect, Inc, Regeneron Pharmaceuticals, SELLAS Life Sciences Group: Research Funding; Delta-Fly Pharma, Inc.: Research Funding; SELLAS Life Sciences Group: Research Funding; Glycomimetics: Research Funding; Hikma Pharmaceuticals: Speakers Bureau; Janssen Research and Development: Research Funding; Novartis: Consultancy; Daiichi Sankyo, Genentech, Inc., Genzyme, Jazz Pharmaceuticals, Liberum, Novartis, Pfizer, PinotBio, Inc, Pulmotect, Inc, Sanofi-Aventis, Servier: Consultancy; Regeneron Pharmaceuticals: Research Funding; AstraZeneca: Research Funding; AbbVie, Amgen, Inc, Ascentage Pharma Group, Astellas Pharma Global Development, Astex, AstraZeneca, BMS, Celgene, Cellenkos Inc, Cyclacel, Delta-Fly Pharma, Inc, Genentech, Inc., Genfleet, Glycomimetics, Iterion, Janssen Research and Development: Research Funding; Amgen, Inc.: Research Funding; Celgene: Research Funding; Genentech: Consultancy, Research Funding; Iterion: Research Funding; Sanofi-Aventis: Consultancy; Biologix, Cure, Hikma Pharmaceuticals: Speakers Bureau; Pulmotect, Inc.: Consultancy, Research Funding; Astellas Pharma Global Development: Research Funding; Pfizer: Consultancy, Research Funding; Servier: Consultancy; Pinotb-Bio: Consultancy; BMS: Consultancy, Research Funding; Astex: Honoraria. Daver:Astellas: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Syndax: Consultancy; AROG: Consultancy; Kronos Bio: Research Funding; Glycomimetics: Research Funding; Hanmi: Research Funding; Trovagene: Research Funding; Shattuck Labs: Consultancy; Novimmune: Research Funding; FATE: Research Funding; Servier: Consultancy, Research Funding; Novartis: Consultancy; Celgene: Consultancy; Agios: Consultancy; Pfizer: Consultancy, Research Funding; Jazz: Consultancy; AbbVie: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Kite, a Gilead company: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding. Konopleva:AbbVie, Forty Seven, Precision Biosciences, Gilead Sciences, Genentech, Janssen, Sanofi, MEI Pharma, Daiichi Sankyo Pharmaceutical, AstraZeneca Co., Menarini.: Consultancy; Reata Pharmaceuticals.: Current holder of stock options in a privately-held company, Patents & Royalties; Abbvie, Allogene Therapeutics, Cellectis, Forty Seven, Gilead Sciences, Genentech, Sanofi, MEI Pharma, Rafael Pharmaceuticals, Daiichi Sankyo Pharmaceutical, AstraZeneca Co., Menarini, Precision BioSciences.: Research Funding. Kantarjian:Pfizer: Research Funding; Novartis: Research Funding; Jazz Pharma: Research Funding; Orsinex: Honoraria; Daiichi-Sankyo: Research Funding; Cyclacel: Research Funding; BMS: Research Funding; Actinium: Honoraria; Astex: Research Funding; Immunogen: Honoraria, Research Funding; Ariad: Research Funding; Amgen: Honoraria, Research Funding; Agios: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Takeda: Honoraria. Ravandi:Syros: Consultancy, Honoraria, Research Funding; Xencor: Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Biomea fusion: Honoraria, Research Funding; Prelude: Research Funding; Astex/taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding.
ASTX727 is not currently approved for AML.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal